Nothing brings friends and family together like a delicious, memorable dessert. Whether it’s cookies for Christmas, King Cake for Mardi Gras, or a beautiful birthday cake, a sprinkle of sparkling sugar crystals on top is the perfect finishing touch on any baking project.
The beauty of sugar crystals is also apparent when viewed under a microscope. To the naked eye, dry refined sugar looks like tiny cubes, but at starting at a magnification of 40x, their wonderfully distinctive oblong, column-shaped forms come into view.
The chemical formula of sugar is C12H22O11, which means each molecule contains 12 carbon atoms, 22 hydrogen atoms, and 11 oxygen atoms. The chemical name for sugar is sucrose.
“Table sugar is a disaccharide made when two monosaccharides, specifically fructose and glucose, bond together,” said Leanne Cope, a science teacher at Aurora High School in Aurora, Missouri.
Like all compounds made from these three elements, sugar is a simple carbohydrate. Sugars are further broken up into monosaccharides and disaccharides, with “mono” meaning one sugar unit and “di” meaning two sugar units bonded together, according to Cope. Sugar crystals are formed when highly ordered arrangements of these sugar molecules are attracted to each other and bond together in a repeating pattern.
Many fruits, nuts and vegetables naturally contain sucrose, with some containing as much as 10% sucrose. However, nothing tops sugarbeets and sugarcane which contain about 16 and 14%, respectively, making them the most efficient way for farmers to grow and harvest sucrose. After sugarbeets and sugarcane are harvested, they are sent to mills and factories. The sugar is extracted from sugarbeets by diffusion with hot water while sugarcane is chopped and crushed. The resulting sugar juice syrup is carefully boiled and seeded with microscopic sugar crystals to start the crystallization process.
“Crystallization occurs when you have a supersaturated solution of a substance, and you allow the water to evaporate out of that solution. A supersaturated solution forms when you heat a solution to dissolve more of a particular substance,” Cope said. “An example would be a simple sugar syrup solution used when making sweet tea or homemade lemonade. When you heat the water in a sugar solution, you are ‘forcing’ it to dissolve more sugar than it would at room temperature. As the water evaporates, the sugar molecules will begin to crystallize by arranging themselves in repeating patterns, resulting in a solid.”
When the crystals reach their desired size, a rich mixture of crystals and molasses syrup is formed. The sugar crystals are then separated from the syrup and are now 99.9 percent pure white sugar. These crystals move into the granulator, a machine that turns raw materials into uniform granules, where they are dried, cooled, and separated according to size. Sugars are also classified by crystal size, such as granulated, powdered, and superfine. Powdered sugar, also known as confectioner’s sugar, has the smallest crystals due to being finely ground. Superfine sugar also has a small crystal size and is generally used in making delicate desserts like mousse or puddings. Because the crystals are so fine, they dissolve easily, even in cold drinks. Granulated sugars have medium-sized crystals, and the largest crystals are found in sanding, demerara and turbinado sugars.
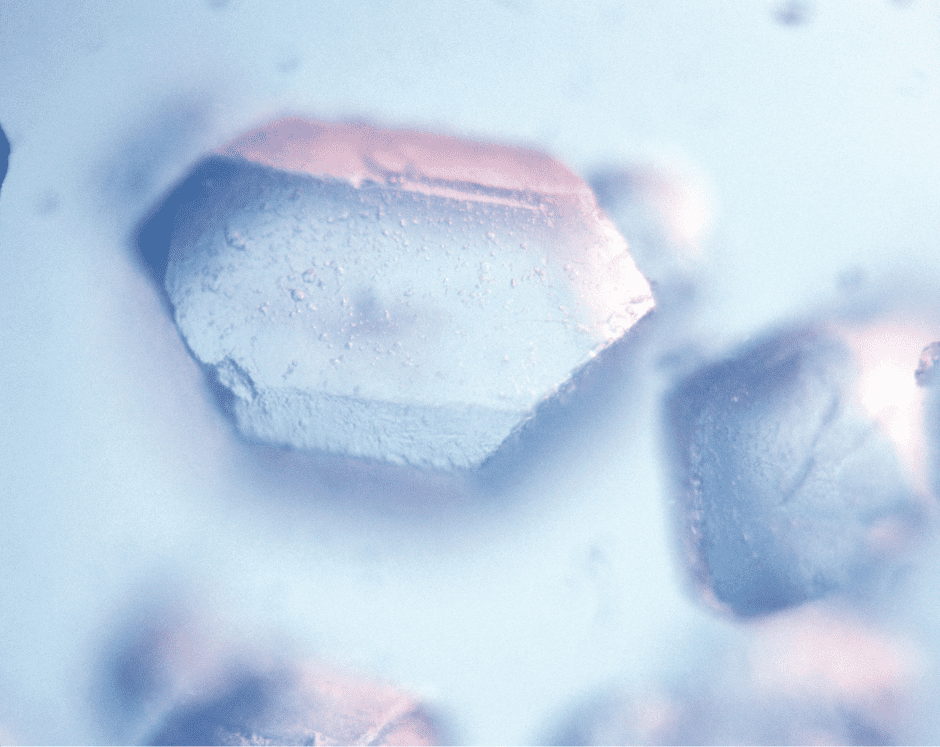
Sugar crystals at 100x magnification.
“One of the things that makes crystals so interesting is that different molecules arrange themselves in different patterns,” said Cope. “Table salt tends to be very cube-like in shape, quartz forms a hexagonal prism, and snowflakes form a hexagonal ring shape. Sugar crystals are an elongated cube that slants to one side, like a leaning rectangle-shaped cardboard box.”
The fact that sugar solidifies into crystals is very important in candy making, and candy can be categorized as crystalline or non-crystalline. Non-crystalline or amorphous candies such as lollipops, taffy, and caramels do not contain crystals. Recipe ingredients and procedures for non-crystalline candies are specifically designed to prevent the formation of sugar crystals, because they give the resulting candy a grainy texture.
Candies in the crystalline category contain sugar crystals in their finished form, such as fudge and fondant. Rock sugar, also known as rock candy or sugar candy, is a hard confection made by cooling sugar syrup into large crystals. This treat is as educational as it is tasty.
“Growing rock candy is a perfect way to experiment with precipitation and crystallization,” said Cope. “Precipitation is when a solid is “pulled” out of a solution. When making rock candy, the sugar molecules are separated from the water molecules as the water cools and evaporates, allowing them to form crystals.”
The key to successfully making rock candy is to make sure the sugar in the solution is completely dissolved and appears clear, according to Cope.
“You want as many sugar molecules in it as possible. Also, don’t forget to roll your string or stick in sugar after you dip it in the solution because this gives the dissolved sugar in your solution a place to crystallize,” she said. “This is ‘seeding’ your crystals. Also, air humidity plays a significant role in being successful at growing rock candy. You need the water to evaporate out of your solution which is harder to do when the humidity is high.”
Next time you decorate cakes and cookies or sit down to a delicious dessert topped with sugar crystals, take time to enjoy the “sweet” results of chemistry in action.










Get Social with #MoreToSugar